Biosimilar medicines, a nearly identical copy of an original biologic treatment, have recently taken center stage in the pharma industry. As approvals and implementation of biosimilar products, therapies, and medicines continue to accelerate, the U.S. healthcare system is set to experience enormous cost cuts and save Americans millions of dollars in life-essential care. The rise of the COVID-19 pandemic in April 2019 ushered in a historic wave of 58 approvals by the European Medicines Agency, and activity in biosimilar production hasn’t slowed since.
The financial burden of manufacturing biological medicines involves using cutting-edge technologies to extract large complex molecules derived from living cells—a challenging and expensive process. On the other hand, biosimilar manufacturing relies on replicating the amino acid sequences of its reference drug, leaving a small window for minor differences and eliminating the costs and lead time for FDA approval.
Already, pharma investors are reaping the financial benefits of pouring capital into the cost-effective alternative. For biological medicines, studies have revealed research and development costs at $985 million to bring a single product to market. In addition, patients experience the financial brunt, paying on average between $10,000-$30,000 per year for some treatments, with some reaching millions of dollars, according to the National Conference of State Legislature.
Contrarily, biosimilars have list prices 15% to 35% lower than their reference products, saving individuals $8 billion in 2020 alone. As patents expire and new biosimilar products are introduced, savings in 2022 are projected to surpass $30 billion and drive price competition within the pharma industry.
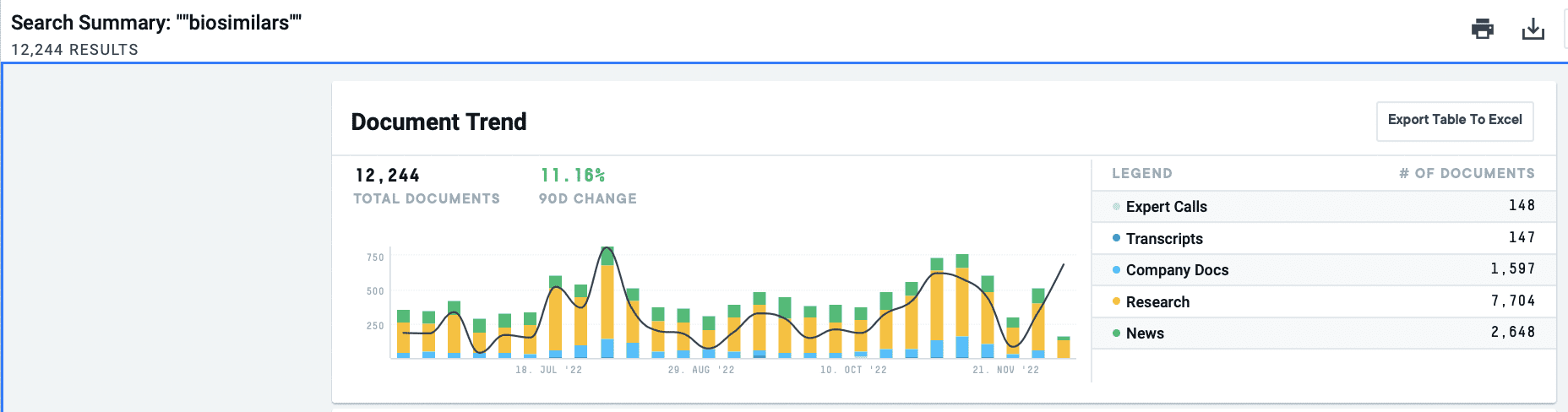
As biosimilars simultaneously shrink healthcare expenditures and rake in investment capital, we took a deep dive into this lucrative industry trend. In the AlphaSense platform, there has been an 11% increase over the last six months of documentation around “biosimilars”. Below, we look at how the Inflation Reduction Act plays into the biosimilars market and what C-Suite leadership from major pharma companies and industry experts are saying.
Related Reading: The Evolution of Pharma Market Research
The Inflation Reduction Act
This past October, biosimilar drug-pricing provisions associated with the Inflation Reduction Act went into effect. Over the next five years, Medicare is expected to financially cover physician-administered biosimilars at an ASP plus 8% rate to foster patient and medical professional consumption.
Prior to the Act, Medicare paid the average sales price plus 6% for a biosimilar product and separately payable drugs—the average sales price consists of the manufacturers’ sales to U.S. purchasers, excluding rebates, discounts, and price concessions. Consequently, the Act drives biosimilar production to develop biological product competition and lower healthcare costs.
Related Reading: How the Inflation Reduction Act Impacts Big Pharma
But the Centers for Medicare and Medicaid Services (CMS) believe that Medicare’s temporary coverage increase will drive much more than just competition between biologic and biosimilar products.
In a statement, CMS Administrator Chiquita Brooks-LaSure shared that it “will foster competition in the drug marketplace for conditions such as diabetes, cancer, and immune disorders.” Since the slow introduction of biosimilars between 2015 and 2018, corresponding therapy uptake has increased so much that originator biologic market shares in the trastuzumab, rituximab, and bevacizumab classes have depreciated.
The top ten Part B drugs expended to Medicare through the buy-and-bill process are all biologics that do not have biosimilar counterparts. However, the Inflation Reduction Act is not the only motivator behind the adoption of biosimilars. Research has revealed growing trust among physicians and patients alike with biosimilars thanks to education campaigns and improved regulatory guidance from the Food and Drug Administration.
“As the latest government policy projected to inject competition among biologics, add-on payments for biosimilars may produce additional modest gains in market share by these products,” Forbes analyst Joshua Cohen writes.
The Outlook on Biosimilars
Equity research found within the AlphaSense platform aligns with predictions proposed by industry experts. The biosimilar market size will grow by an increasing number of biological losses of exclusivity (LOE). LOE describes the transition period when once a drug’s patent expires, the manufacturer must relinquish control of the drug formula, allowing manufacturers to produce biosimilars.
As more biosimilars enter the market, analysts see the potential for a greater drug channel split between medical vs. pharmacy benefits. Physicians are likely to prescribe more biosimilars with increasing widespread acceptance and integration. And with increases in biosimilar uptake, additional therapeutic areas will likely advance, focusing on retina diseases, bone health, G.I., and derm and, consequently, increase the cost of biosimilar development.
These industry predictions are leaving pharma manufacturers and investors scrambling to decipher what and where lies the greatest opportunity for reaping the benefits of biosimilar production.
Biosimilars Growth Areas
There seems to be no shortage of opportunities for pharmaceutical manufacturers to create biosimilar products that treat a wide range of ailments and conditions. Ranging from hormone replacement therapy to immunotherapy, insulin shots, breast cancer, and even brain disorders, every biological product approaching LOE has the potential to be a cash-cow opportunity for the quickest and smartest investor.
Additionally, company leadership is bringing a lens to the national and international markets and their corresponding approval processes to deduce where the most financially ripe biosimilar opportunities are. The U.S. imposes lengthy FDA processes for some medications, even though trust in biosimilars is rapidly growing. Meanwhile, the EMEA market has already embraced biosimilars with shorter approval turnarounds and greater prescription rates.
Below, we dig into how pharma C-Suite leadership is designating and pursuing growth areas for biosimilars. By scanning thousands of expert transcripts, earning calls, and various company documents in our extensive content library, we were able to decipher where you can expect to see the most activity in the coming fiscal quarters.
Key Commentary on Biosimilars Growth Areas
Expert Call Transcripts Commentary
“On the market side across Europe, the biosimilars roughly represent 10%, 12% of the total biologics market and it is up from 2% only five years ago, so it’s a pretty nice penetration, and of course, the main growth drivers have been oncology and immunotherapy. Also, if you look at the therapeutic area coverage, then probably 2/3 of the biologics market value is accessible to biosimilars already and has been accessed. This is perchance that oncology and immunotherapies were the first to expire, therefore, 3/4 of the sales are actually happening in those two therapeutic areas. I would imagine that this remains the same in terms of the trend of higher coverage.”
– Former Director of STADA on oncology and immunotherapy being major growth areas for biosimilars | Expert Call
“AbbVie is going to face biosimilar competition in a couple of years for BOTOX, which is one of the most important growth drivers, one of the reasons to acquire Allergan. The current treatments only just improve the symptoms and try to delay the progression, but it’s really important for Parkinson’s Disease patients to slow the progression. BOTOX is a really important driver for AbbVie’s long-term growth. That’s why we were working on the four different indications to await the biosimilar competition.”
– Former AbbVie Associate Director for Pipeline Commercialization on the importance of biosimilars for BOTOX to treat Alzheimer’s and Parkinson’s Disease | Expert Call
“The other thing that Amgen is doing really well is positioning their biosimilar portfolio as being from the manufacturer that basically invented biologics. They know how to manufacture biologics. They have a strong history of quality. They don’t have recalls in their pipeline. They’re positioning it as it’s coming from a manufacturer that you’ve trusted for 25 years. If you’re going to use a biosimilar, why wouldn’t you use one that is from the company that started biologics?”
– Former Novartis Director of Rheumatology Marketing on biological companies taking advantage of biosimilar production and marketing | Expert Call
“If I was working for a biosimilar company, firstly, they would need to understand that the insulin market is now flat because we are investing in a different type of products. Of course, if a biosimilar needs to penetrate, it needs to present itself with a strong scientific message and create reputational confidence among the physicians. Based on those types of behaviors from the biosimilar companies, I don’t see them as a threat right now. However, should they change their approach and way of working in terms of doing business, of being more scientific in their way of working and making sure that they have enough stock, then things could be different.”
– Former Novo Nordisk Commercial Affairs Director on biosimilar integration into once-weekly insulin shots | Expert Call
“In terms of relaxation, actually now, if you consider approval timeline, for example, U.S. versus Europe, U.S. almost meantime to get approval is around 1.3 years, whereas in Europe you have 210 days to get approval for a biosimilar. Of course, it depends on the type of molecule and the complexity involved, but the regulatory framework in the E.U. is much more flexible, and industries are coping with the requirements. I don’t see any further relaxation. Expect new evaluations and technologies rapidly coming. Maybe further down the line, we see some changes in the guidance.”
– Current Unit Head of Milpharm Limited on E.U. being a more flexible market for biosimilars than the U.S. | Expert Call
Earnings Call Commentary
“Outside the U.S., biosimilar pricing tends to come down fairly rapidly and then can hold in some of the larger, what we call retail markets. In markets where it’s a heavy tender business, prices will continue to decline as long as there are competitors in the market.”
– AMGEN INC. Q3 2022 Earnings Call
“The most significant growth driver has been our 351(k) interchangeable biosimilar insulin largely in the U.S. market, which has attained a double-digit market share at the end of this quarter.”
– Biocon Ltd. Q4 2022 Earnings Call
Press Release Commentary
“The company’s pegfilgrastim biosimilar is a supportive care medicine for patients with non-myeloid cancer. It stimulates the growth of certain white blood cells, which are essential to prevent or fight infections, a common life-threatening risk in patients receiving myelosuppressive chemotherapy.”
– Fresenius Kabi AG Press Release
“Our current biosimilars portfolio targets autoimmune disease, eye disorders, bone disease, respiratory disease, and cancer. A biosimilar to Humira (adalimumab) is already approved in Europe (Hukyndra) and Canada (Simlandi), and three biosimilar candidates, including AVT03 have entered or completed confirmatory patient studies.”
A Need for Greater Biosimilars Awareness and Education
Mistrust for biosimilars appears to still be omnipresent. A recent study from Vita-Salute San Raffaele University and Humanitas University highlights concerns among patients regarding the prescription of biosimilar drugs—despite their proven safety and cost-effectiveness. Findings cite a significant lack of knowledge among gastroenterologists around the effectiveness, safety, and complexities of switching to biosimilars for treating inflammatory bowel disease (IBD).
But this issue is not new: a 2017 survey involving international physicians, including gastroenterologists, confirmed knowledge gaps related to biosimilars, mentioning specific difficulties defining terms like “extrapolation” and “interchangeability.” Due to this lack of understanding, the study aimed to assess gastroenterologists’ attitudes toward biosimilars and their prescription practices.
The research, conducted as a global cross-sectional study between January and February 2023, involved 234 physicians from 38 countries. Most respondents were highly experienced gastroenterologists (86%), and the majority worked in settings caring for substantial numbers of IBD patients. A significant finding was that 83.3% of participants believed biosimilars to be as effective and safe as originator drugs, while only 3.9% had reservations about their effectiveness and safety.
For 2024 and beyond, awareness and education for biosimilars will be necessary for drug adoption, enhancing long-term patient care, and prescription cost-efficiency.
Biosimilar Manufacturing Competition Brewing in 2024
In 2023, the global biosimilar market generated approximately $21.2 billion, leading IMARC Group to anticipate a $164.5 billion evaluation by 2032 or a compound annual growth rate (CAGR) of 25.1% from 2024 to 2032. So how are companies preparing themselves for this immense market opportunity? The answer is establishing internal production and manufacturing methods so as to ensure reliable and secure forms of delivering biosimilar supply.
In-house production provides a biosimilar manufacturer comprehensive control over product quality—a critical factor for biosimilars. Due to the intricate nature of biologics and the stringent regulations governing their production, companies typically opt for internal methods (versus contract-manufacturing) to uphold elevated standards.
Additionally, biologic manufacturing processes often incorporate proprietary methods and technologies, so opting for in-house production safeguards these trade secrets, making it the favored approach. Despite the potentially high initial setup costs, maintaining production in-house can lead to substantial reductions in per-unit costs over time.
In-house manufacturing also allows companies to mitigate the risk of supply chain disruptions, ensuring a steady supply of their products. This approach also promotes flexibility and agility in production, facilitating adjustments or adaptations to the product or process as required.
Based on equity research found within the AlphaSense platform, Fresenius Kabi, Hikma, Gedeon Richter, Gerresheimer and Sandoz (under the Novartis umbrella) all seem ripe for taking advantage of biosimilar growth.
Fresenius Kabi boasts one of the most extensive biosimilar pipelines, comprising approximately 15 assets, closely followed by Gedeon Richter and Hikma. Notably, there is significant convergence in biosimilar exposure to Humira (with peak sales reaching USD 21.2 billion), Stelara (reaching USD 10.3 billion in peak sales), and Denosumab (peaking at USD 6.9 billion) over the next three years.
Fresenius Kabi is strategically positioned to initiate the initial wave of launches for three of its biosimilars, including tocilizumab, which is anticipated to reach USD 3 billion in sales as early as H1 2024. These launches have the potential to substantially impact Kabi’s profitability and margin, signifying a pivotal development for the company.
Both Gedeon Richter and Hikma have exposure to denosumab but may introduce this product in the second wave of launches. Gedeon Richter has already successfully commercialized two biosimilars, Bemfola and Terrosa. Meanwhile, Hikma has established a robust presence in oncology biosimilars through license agreements in key Middle Eastern markets.
Biosimilars currently constitute less than 5% of group revenues for both companies. Gedeon Richter reports losses in this segment, primarily attributed to substantial R&D spending. In contrast, biosimilars are contributing incrementally to Hikma’s group margins, based on our assumption.
Currently, Sandoz lacks internal biosimilar manufacturing capabilities and relies on Novartis for its supply chain. The majority of the supply chain for small molecules is outsourced, except for antibiotics, especially APIs for penicillins, and certain complex generics.
However, Sandoz holds the position as the world’s largest manufacturer of generic antibiotics by volume, and it possesses the last fully integrated production facility in Western Europe, situated in Kundl, Austria. Alongside its extensive antibiotic portfolio, which includes over 150 products covering more than 50% of available antibiotics, Sandoz is also involved in producing active pharmaceutical ingredients (API) for external companies.
Tracking Pharma Developments in Real-Time
Identifying and pursuing pharma industry trends can be a full-time endeavor, as new biosimilar products and approval processes are constantly being introduced to the market. It’s why more C-Suite executives are implementing market intelligence platforms that leverage AI into their operations to quickly find the answers they need to act confidently and swiftly. AlphaSense provides this and more.
Sort through our extensive content library, aggregating business documents from over 10,0000 content sources, using our AI search technology, and be at the forefront of your industry.
Start your free trial with AlphaSense today to see how our platform can get you ahead of your competition.