10 Market-Moving Trends to Shape 2023
Get the guide
Since the onset of the COVID-19 pandemic, the pharma industry has experienced rapid, radical change. From billion-dollar funding for research on widespread health adversities to manufacturing new technologies to address them, the virus has ramped up lucrative investment opportunities in nearly every industry sector. And there’s no sign of market activity slowing anytime soon, as geopolitical factors and variants of COVID-19 continue to open new doors for funneling capital.
But this financially prosperous time for pharma companies has left C-Suite leadership and stakeholders wondering: what does the future of healthcare entail? Deciphering future diseases and potential treatments is imperative to distinguish between a worthy investment or financing an industry fad. That’s why more and more pharma companies are leveraging market intelligence platforms to see what their competition, Wall Street, and the industry is saying and to find the answers they need.
Using the AlphaSense platform, we dug into the pharma trends likely to dominate 2024 and beyond. Below, learn how digital transformation, artificial intelligence (AI), the adoption of big data, and much more are redefining what medicine looks like for both patients and providers.
How the Pharmaceutical Industry is Changing
When the pandemic began in 2020, the pharmaceutical industry faced a series of challenges. COVID-19 changed the conditions in which Americans were willing to go back or even continue working, with many reconsidering their career paths. In 2021, more than 47 million workers quit their jobs while 32% of Americans switched professions, ushering in a wave of unemployed workers and, consequently, the Great Resignation. This alone has pushed pharma companies to reconsider how they can fulfill demand without the use of human workers.
Additionally, the Russia-Ukraine war has extensively affected global supply chains. Essential commodities that many sectors rely on are no longer guaranteed, forcing suppliers to reconsider how they can acquire what they need to meet their end users’ demands. As the strain on materials continues to grow in this conflict, the monetary deficit brought on by this shortage has ushered in staggering inflation prices. According to a report by the Department of Health and Human Services, drug prices on more than 1,200 prescription drugs rose faster than inflation between July 2021 and July 2022, rising on average 31.6%.
These market-disrupting events have forced pharmaceutical companies to strategize how providers can continue to treat without a steady supply of materials or human talent. The results: medical processes streamlined with the use of machine learning, treatments administered and monitored in digital spaces, artificial intelligence playing an increasing role in our healthcare systems, and much more.
Related Reading: The Evolution of Pharma Market Research
Recent Legislation Shaping the Industry
Healthcare systems globally are undergoing significant reforms, impacting drug manufacturers through stricter access and pricing regulations. Specifically within the U.S., the government has initiated negotiations for the first 10 products under the Inflation Reduction Act (IRA).
Through the IRA, Congress seeks to make prescription drugs more affordable with reforms that will reduce the price of drugs and limit out-of-pocket costs for many Medicare patients. In today’s healthcare reality, prescription drugs account for about 20% of Medicare patients’ out-of-pocket costs. Spending on prescription drugs continues to grow as other health spending has decreased.
But when the initial IRA negotiations took place last year, the pharma industry responded by launching a series of new lawsuits that invoke the First and Fifth Amendments. Merck & Co. and Bristol Myers Squibb, for instance, are challenging the IRA’s constitutionality. Meanwhile, lobbyists and their co-litigants are incorporating the Eighth Amendment into their lawsuit.
According to a Pharma Voice article, William Soliman, CEO and founder of the Accreditation Council for Medical Affairs, views this approach of litigation as strategic. “They are using a multi-pronged approach in tandem,” he says. “The purpose (is) to hit the government on all ends and to ultimately force them to overturn or seriously limit the impact the IRA can have.”
Meanwhile, proposed EU legislation aims to reduce the exclusivity window for new drugs from 10 to eight years if they do not launch in all 27 member states within two years. Germany is also considering reforms to its pharmaceutical regulations (AMNOG 2.0) to control drug costs, while Italy is restructuring the Italian Medicines Agency (AIFA). In Japan and China, similar efforts are underway to lower drug prices. These reforms collectively signal a period of uncertainty and potential revenue pressure for the pharmaceutical industry.
Trends Dominating the Future of Pharma
COVID-19 renewed interest in modernizing healthcare, as an increasing amount of investor attention is being spent on scouting for future growth areas for pharma. Moreover, weaknesses that existed in medical systems before the pandemic have refreshed concern from companies, stakeholders, and the like to devise solutions to overcome them.
In addition to regulatory and national legislation (i.e., The Inflation Reduction Act, which mandates Medicare to financially cover physician-administered biosimilars at an ASP plus 8% rate) to driving stakeholder engagement, competition couldn’t be thicker to find the next big vaccine, management system, or therapy.
Vaccines
In 2020, Pfizer, Moderna, and Johnson & Johnson competed to create the first effective COVID-19 vaccine.
While all served the same purpose of immunizing recipients against the virus, conversations emerged about which vaccine outperformed the others. This inevitable need for consumers to compare and rank treatments has only pushed pharma companies to invest in crafting an industry-changing vaccine. And recent news shares promise of a future where some of the world’s leading causes of death (i.e., cancer, HIV, etc.) are eradicated with a single, or a series, of shots.
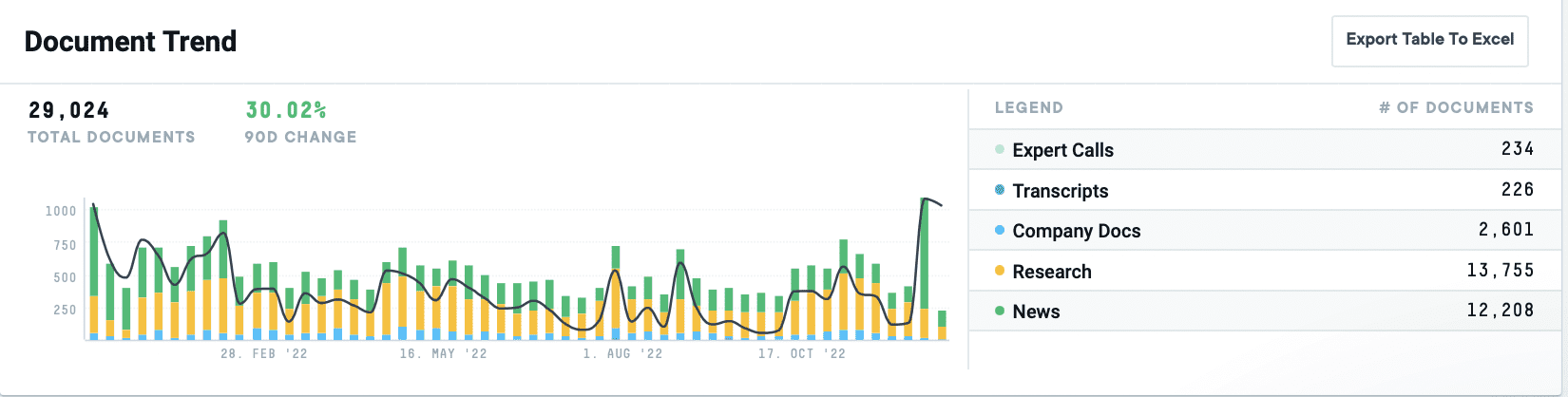 Caption: AlphaSense shows a 30% increase in aggregate documents mentioning “vaccines” over the last 12 months
Caption: AlphaSense shows a 30% increase in aggregate documents mentioning “vaccines” over the last 12 months
Pfizer has stirred up vaccine excitement with their recent announcement to launch five “new vaccines,” plus two new medicines in oncology and immuno-inflammation. Meanwhile BioNTech, which collaborated with Pfizer to create its COVID-19 vaccine, said immunization against cancer could be possible as soon as 2030. Experts agree that pharma companies who’ve had a leg in the COVID-19 vaccine race are set up for future vaccine-crafting success.
Artificial Intelligence
Mentions of AI applications within healthcare are steadily rising within the AlphaSense platform, especially within our library of patent applications and clinical trials. Physicians, surgeons, and nurses are leveraging artificial intelligence to retrieve insightful analytics that drive care optimization and workflow efficiency through predictive, AI-powered systems.
Likewise, pharma manufacturers are sharing how they’re integrating AI in telemedicine and imaging to treat various conditions, from diabetic retinopathy to blood cancer and even internal bleeding. And to aid providers, pharmaceutical manufacturers are incorporating AI to assist medical professionals in their patient consultations (i.e., AI voice-activated assistants to transcribe doctors’ notes).
In the eyes of medical manufacturers, there seem to be endless possibilities for how AI can be woven into healthcare.
“Applying AI to big data in life sciences can help companies reshape business models, streamline biopharma manufacturing, and enhance everything from cognitive molecule research and clinical trial data flow to self-healing supply chain applications and product intelligence,” Deloitte stated. “It can also enable life sciences companies to be more personalized and authentic in how they engage with health care professionals, patients, and other stakeholders.”
Machine Learning
Machine learning has widely expanded the capabilities of not only medical manufacturers but providers. The COVID-19 virus propelled the concept of replacing human workers with robots—mechanical workers that operate on “machine learning” or the application of AI that enables systems to learn and improve from experience without being explicitly programmed.
Patents and clinical trials found within the AlphaSense platform have been a steady, reliable source of information on how robotics are continuously revolutionizing the ways in which doctors diagnose and even prescribe.
Machine learning in pharma focuses not on replacing a doctor but on enhancing their medical expertise using artificial intelligence programs. By combining the knowledge that a physician has and the vast amount of data available to medical professionals–ranging from new drugs to disease symptoms, drug interactions, and patient outcomes—machine learning can provide deeper insight into more precise patient diagnoses and, consequently, treatment. Already, doctors are using AI programs in x-ray readings and CT scans to identify suspicious nodules and lesions in lung cancer patients.
Big Data
Based on equity research found within the AlphaSense platform, industry experts expect big data to have the greatest impact on the pharmaceutical industry.
In addition to AI, big data is providing pharmaceutical companies with predictive, prescriptive, and diagnostic analytics that have led to new drug discoveries and developments. Further, it allows scientists to work with and link large datasets, detect patterns in real-time, predict outcomes, undertake dynamic risk scoring and test hypotheses. By applying these strategies to better inform decision-making, healthcare systems can leverage big data to improve the efficiency of research and clinical trials, building new tools for physicians, patients, and even regulators.
Documentation in the platform reveals that, currently, multiple healthcare companies, ranging from multi-provider groups to single-physician offices, are adopting big data analytics to also maximize revenues, increase personalized patient care services, quickly yet effectively detect healthcare fraud, and review clinical trials and medical records.
In addition, big data has helped reduce some of the astronomical costs and drawbacks associated with clinical trials.
JAMA reports that the estimated research and development spent to develop a new drug ranges around $1.1 billion, including the costs of failed clinical trials. That’s why recruiting qualified candidates for a clinical study is essential to maintaining a budget. Big data can eliminate the guesswork and time constraints to find the right participants by leveraging genetic makeup, disease status, patient data, demographics, past clinical trial data, and much more.
Gene Therapy
2022 proved to be a major milestone for the global gene therapy space, as a slew of innovative treatments for chronic diseases were launched.
In August of 2022, bluebird bio’s Zynteglo, a one-time treatment that addresses beta-thalassemia at the genetic level, was approved for use within the US by the FDA–the first approval by the administration for gene therapy in over three years.
Internationally, the European Medicines Agency also approved a major gene therapy. PTC Therapeutics’ Upstaza will treat children and adults with severe aromatic L-amino acid decarboxylase (AADC) deficiency, an inherited disease that affects the nervous system leading to symptoms that include weak muscle tone and lack of limb movement.
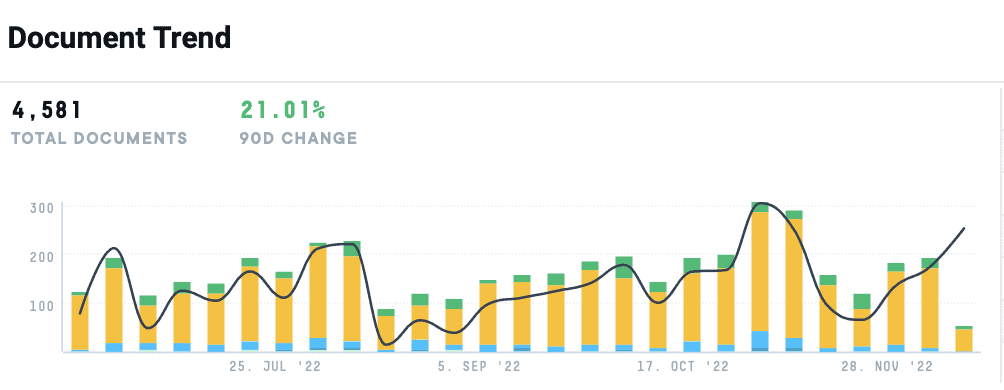
Caption: Over the last six months, AlphaSense has tracked a 21% increase in mentions of “big data” within the pharmaceutical industry
Drug development for disease management also brings a lens to a myriad of pharma supply chain aspects. Investigating scalable and cost-effective production processes, balancing speed-to-market and product quality, as well as tackling delivery and dosage challenges will be the priorities for those getting investing in new gene therapies.
Digital Therapeutics
In the AlphaSense platform, conversations around digital therapeutics being viewed as an accessible healthcare option for global crises–a concern derived from the COVID-19 pandemic–grew in number.
Evidence-based, clinically-approved, and FDA-cleared software that can be accessed through one’s medical device and work in tandem with the prescriptions one’s physician prescribes is filling a gap that one-on-one medical consultations could not. But, by having patients rely on software for medical guidance rather than in-person medical consultations, many are digging deeper into the pathways in which the FDA legitimizes digital therapeutics.
However, experts believe that if digital therapeutics pass through the traditional systems set in place by the administration, then patient and provider trust would likely follow. Eventually, digital therapeutics could become the norm.
“With the FDA, as long as there are clinical trials that are completed and everything is run by people that have experience in the field, as long as there’s enough research and enough science behind it, with the FDA, things should be passed accordingly. That’s how we probably progress in the medical field and how we evolve in terms of ways to continue saving patient lives,” says an expert in one of our Expert Insights calls.
How Can AlphaSense Help You Stay on Top of Pharma Industry Developments?
Pharma industry trends evolve on a near-constant basis, making it a full-time endeavor to decipher which ones to pursue with your attention and capital. That’s why more C-Suite executives are implementing marketing intelligence platforms into their operations to accelerate their research with AI.
AlphaSense’s AI search engine and extensive content library, which aggregates business documents from over 10,0000 content sources, allow our clients to remain at the forefront of their respective industries.
Start your free trial with AlphaSense today to see how our platform can get you ahead of your competition.